Show all atoms bonds lone pairs and formal charges. CO 2 Total 16.
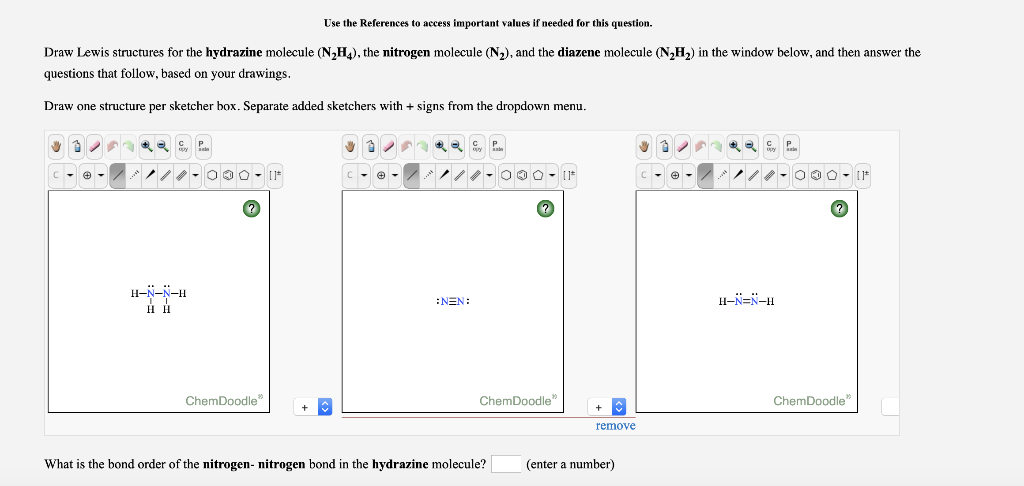
Solved Use The References To Access Important Values If Chegg Com
Follow some steps for drawing the Lewis dot structure of N2H4.
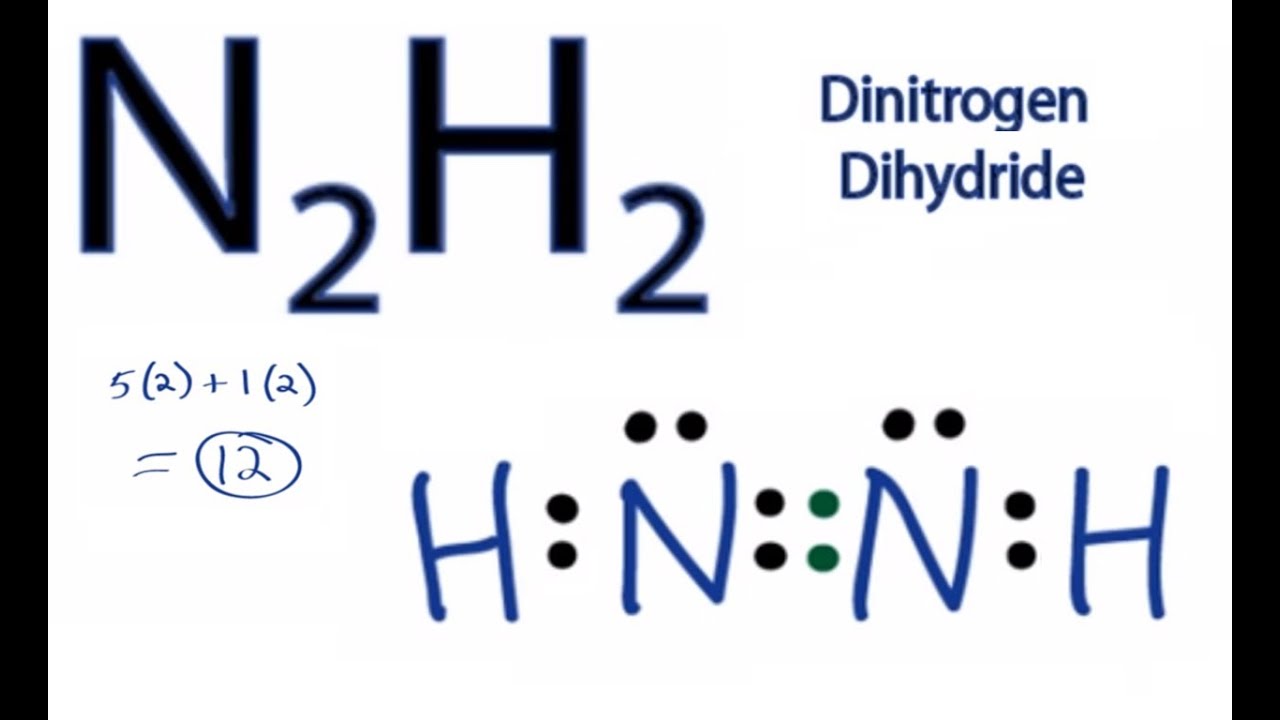
. Given that S is the central atom draw a Lewis structure of OSF4 in which the formal charges of all atoms are zero. You can follow their steps in the video explanation above. Draw the Lewis structures of N_2H_4 N_2H_2 and N_2.
N2 Lewis Structure Setup Its easiest to think in terms of. N 2 H 4 N 2 and N 2 H 2. In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
Clearly label the hybridization of all nitrogen-atoms. For N₂H₄ the two nitrogens are bonded together and with two hydrogens Nitrogen has 5. Draw the Lewis structures of N2H4 N2H2 and N2Draw the molecules by placing atoms on the grid and connecting them with bonds.
Find the shape of the molecule or ion PO3-3. N2H2 Lewis Structure Molecular Geometry Hybridization and MO Diagram. Use the MO model to predict the structure of ketene H2CCO.
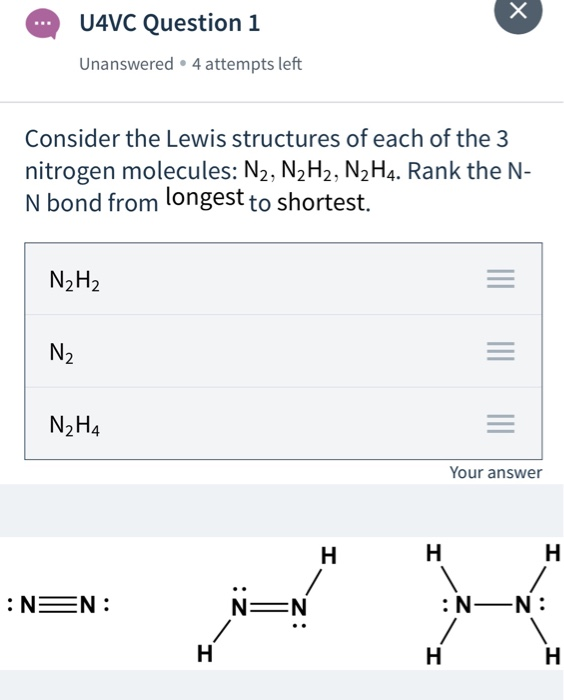
Molecule Bond Angle NH3 1075 N2H4 107 N2H2 120 383 Predict the shape of each molecule. Place least electronegative element in center and draw single bonds from the central atom to other atoms. I need to draw a Lewis Dot Structure for each ion or molecule.
Draw the lewis dot structure for the following molecules ph3. It is a yellowish-colored gas having both cis and trans isomers. 2 Draw the best Lewis structure for NCCH2COCH2CHO a neutral molecule.
We review their content and use your feedback to keep the quality high. In the N 2 H 2 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Draw the Lewis Dot Structure of the molecule or ion CH2F2 D.
Draw the Lewis structures of N. For N₂H₄ the two nitrogens are bonded together and with two hydrogens Nitrogen. If a vessel contains an initial reaction mixture in which N200150 M H200200 M and N2H20000250 M chemistry.
This compound is most commonly known as diazene or diimide. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. How to Draw a Lewis Dot Structure Step 1.
Clearly label the hybridization of all nitrogen-atoms. Draw the Lewis Dot Structure of the molecule or ion OF2 B. It can be prepared from the decarboxylation of azodicarboxylic acid NCOOH2.
A step-by-step explanation of how to draw the N2H4 Lewis Dot Structure HydrizineFor the N2H4 structure use the periodic table to find the total number of. How long does this problem take to solve. Answer to Solved Draw the Lewis structures of N2H4 N2H2 and N2.
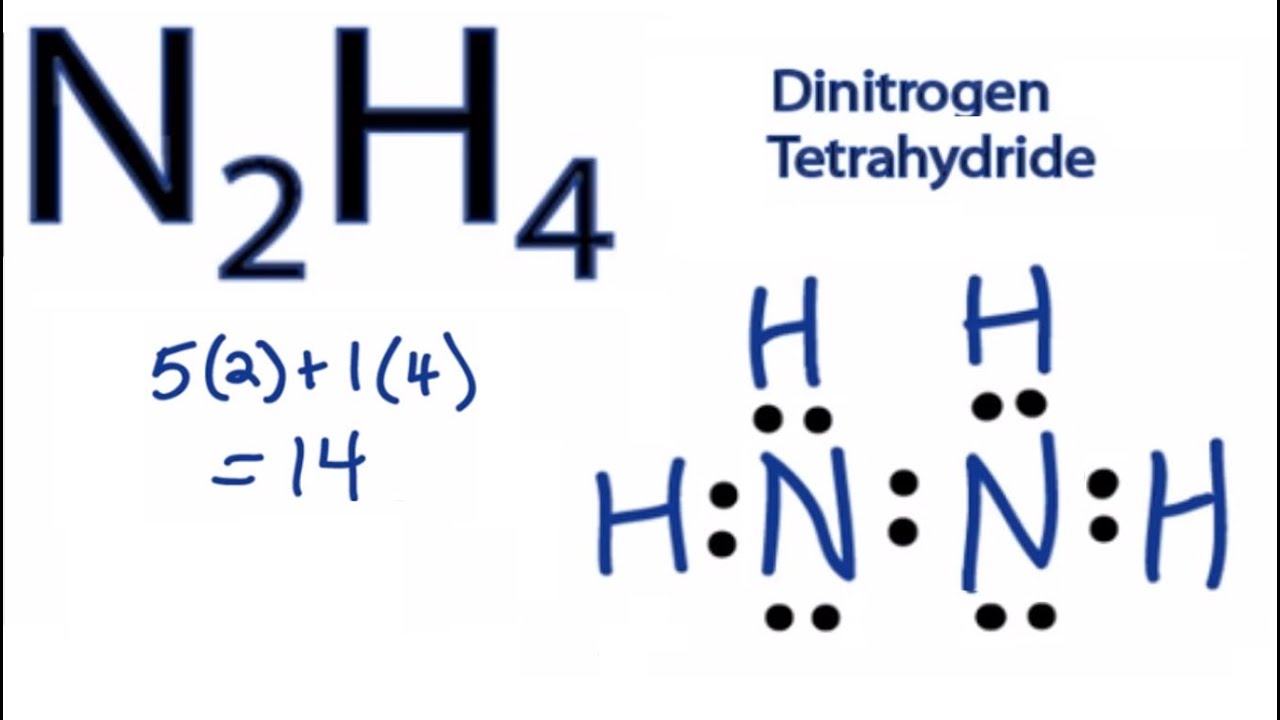
Drawing the Lewis Structure for N 2 H 4. To draw the Lewis structure we must place the central atom with its valence electrons surrounded by the other atoms with their valence electrons then using lines we put the bonds simple line for 1 pair sharing a double line for 2 pairs sharing and triple line for 3 pairs sharing. Determine how many electrons must be added to central element.
Our tutors rated the difficulty of Draw the Lewis structures of N 2H4 N2H2 and N2. A CH4 tetrahedral b PH3 trigonal pyramidal c CHF3 tetrahedral d SO2 bent e SO3 t rigonal planar f CCl2F2 tetrahedral g NH3 trigonal pyramidal hPCl3. Determine the total number of valence electrons to be depicted in the Lewis diagram.
Include all lone pairs of electrons and hydrogen atoms. According to the octet rule nitrogen atoms need to bond three times. N 2 H 2 is straightforward with no double or triple bonds.
Draw the Lewis Dot Structure of the molecule or ion PO3-3 F. Our expert Chemistry tutor Sabrina took 4 minutes to solve this problem. Drawing the Lewis Structure for N 2 H 2.
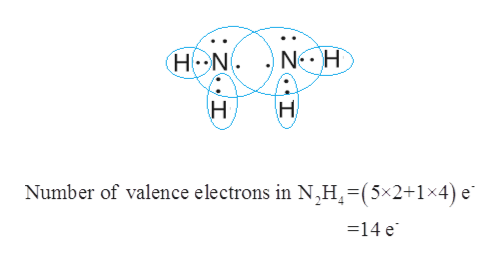
Thus as per the electronic configuration of the element ie. In the Lewis structure for N 2 H 4 there are a total of 14 valence electrons. Science Chemistry QA Library Draw all the lewis structures for N2H2 and N2H4 Which compound has a stronger nitrogen to nitrogen bond.
8 C2H5OH ethanol 9 N2F4. Chemistry students are often confused by the models but drawing Lewis structures can be a straightforward process if the proper steps. Hydrogen H only needs two valence electrons to have a full outer shell.
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule. In the Periodic Table Nitrogen is placed in Group 5 across Period 2.
Find the shape of the molecule or ion OF2 C. Draw the best Lewis structure for NCCH2COCH2CHO a neutral molecule. Include all lone pairs of electrons and hydrogen.
Draw the molecules by placing atoms on the grid and connecting them with bonds. Hydrogen H only needs two valence electrons to have a full outer shell. A Lewis structure also helps to make a prediction about the geometry of a molecule.
Learn this topic by watching Hybridization Concept Videos. As per the molecule N2 it has two atoms of Nitrogen. Which has the shorter nitrogen to nitrogen bond.
25 it has five electrons in its outermost valence shell. Nh3 - n2h4 - n2h2 - c Predict the bond angles about the nitrogen atoms in each molecule. N2 Leweis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms.
Count total valence electron in N2H4. The N2 molecule is diatomic meaning that two atoms of the same element are connected in a pair. Draw Lewis structures for the following molecules.
As we know lewiss structure is a representation of the valence electron in a molecule. Lets start the construction of the N2H4 lewis dot structure step by step-So here we go. In the Lewis structure for N 2 H 2 there are a total of 12 valence electrons.
Find the shape of the molecule or ion CH2F2 E. Dinitrogen dihydride has the chemical formula of N2H2. N 2 H 4 is straightforward with no double or triple bonds.
What is the Lewis dot structure for the HYDRIDE ion. Draw all the lewis structures for N2H2 and N2H4. Which has the shorter nitrogen to nitrogen bond.
To draw the Lewis structure we must place the central atom with its valence electrons surrounded by the other atoms with their valence electrons then using lines we put the bonds simple line for 1 pair sharing a double line for 2 pairs sharing and triple line for 3 pairs sharing. PO4-3 CN- SO3-2 ClO2- N2H2 N2H4 C2H2 C2H4.

N2h4 Lewis Structure Geometry Hybridization And Polarity Techiescientist

Draw The Lewis Structures Of N2h4 N2h2 And N2 Draw The Molecules By Placing Atoms On The Grid And Brainly Com
Answered Draw All The Lewis Structures For N2h2 Bartleby
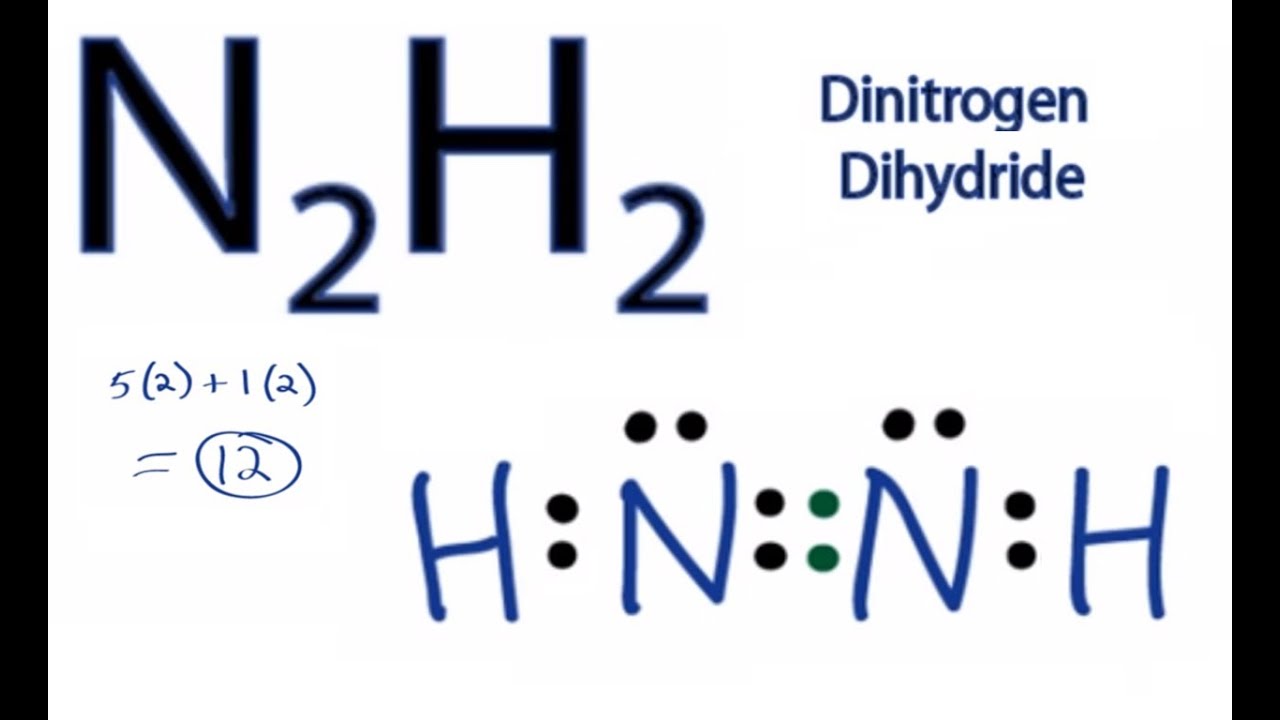
N2h2 Lewis Structure How To Draw The Lewis Structure For Dinitrogen Dihydride Youtube
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

Draw The Lewis Structures Of N2h4 N2h2 And N2 Youtube

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
Solved H U4vc Question 1 Unanswered 4 Attempts Left Chegg Com
0 comments
Post a Comment